Listeria is a Gram-positive bacillus genus in which Listeria monocytogenes appears to be the only human foodborne pathogen specie. The infectious disease associated with Listeria monocytogenes is potentially mortal. Its riskiness is related to the ubiquitous presence of the bacteria in the environment and its growth capacity at low temperature and high salinity. Despite having listeriosis a low incidence (normally less that one in 100,000 people per year) the mortality rate appears to be around 20-30%.
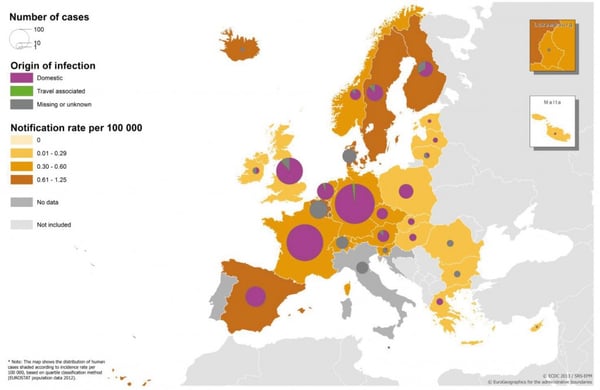
According to the latest EFSA (European Food Safety Authority) report published in 2014 and referred to the 2012 year, there were 1,642 confirmed human cases of listeriosis in the European Union which corresponds to 10.5% increase compared with 2011. The highest notification rates were produced in Finland, Spain and Denmark with Romania representing the lowest notification rate. Almost all the cases were from domestic origin.
Due to the high mortality associated to this pathogen, especially in some population groups, the control of the bacteria by food producers is mandatory. Therefore, the European regulation (EC) No 2073/2005, establishes the criteria for the presence of Listeria monocytogenes in the “ready-to-eat” food. Consequently, food intended for infants and special medical purposes follow the criterion of ‘absence in 25 g of food’.
Besides, in those ready-to-eat food in which Listeria monocytogenes can not grow, the “≤100 ufc/g” criterion at retail level is applied. Finally, in those ready-to-eat foods in which Listeria monocytogenes can grow, the ≤100 ufc/g criterion at retail level is applied together with the “absence in 25 g” criterion applied at processing plant level.
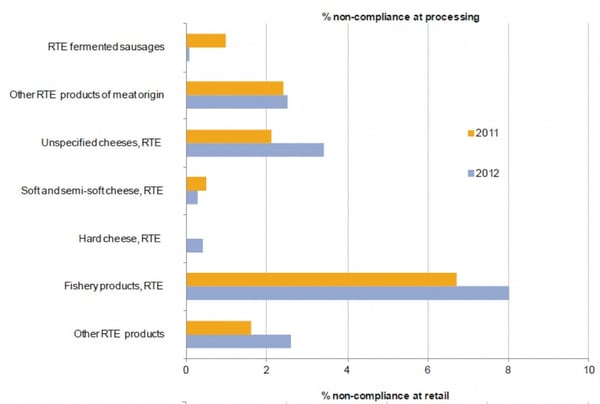
According to the 2012 figures from the EFSA report, the percentage of non-compliance ready-to-eat food at processing varies between 0 and 8%.
Manufacturers of ready-to-eat food have a necessity to reduce the time before a new batch may be released to the market due to the short self-lives of this kind of products. In order to achieve a high level safety confidence and to reduce the manufacturing cost and stocking, rapid methods that can be used in situ offer a considerable advantage against traditional microbiological tests
During the last years ZEULAB has been working in the development of a new generation of tests for foodborne pathogen detection, being Listeria monocytogenes one of them. These tests are based on the concept of “Lab-in-a-box”, in which all the elements necessary for performing a microbial analysis, are integrated in a single device. This device can be used by non qualified staff and without the need of a laboratory.